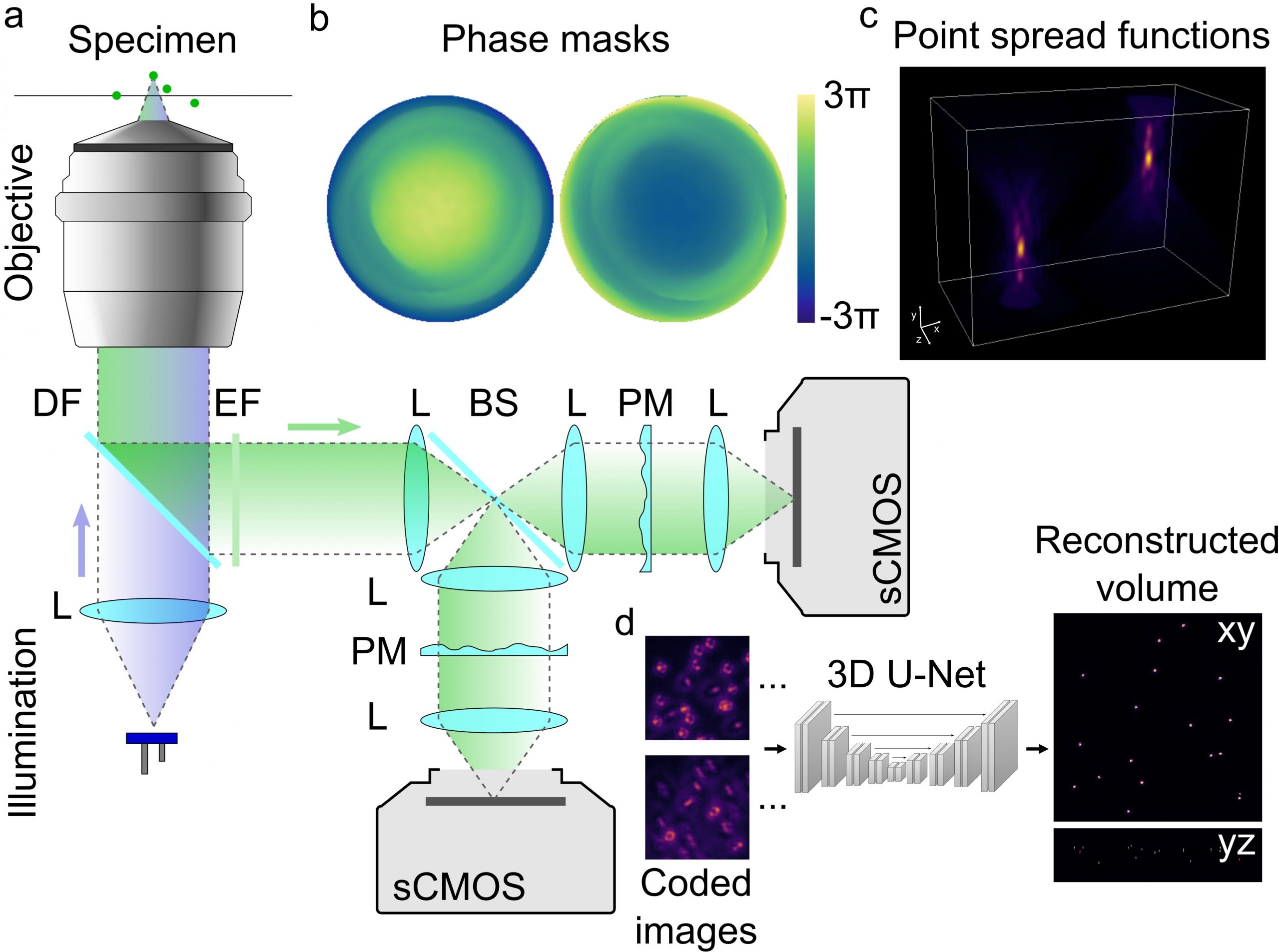
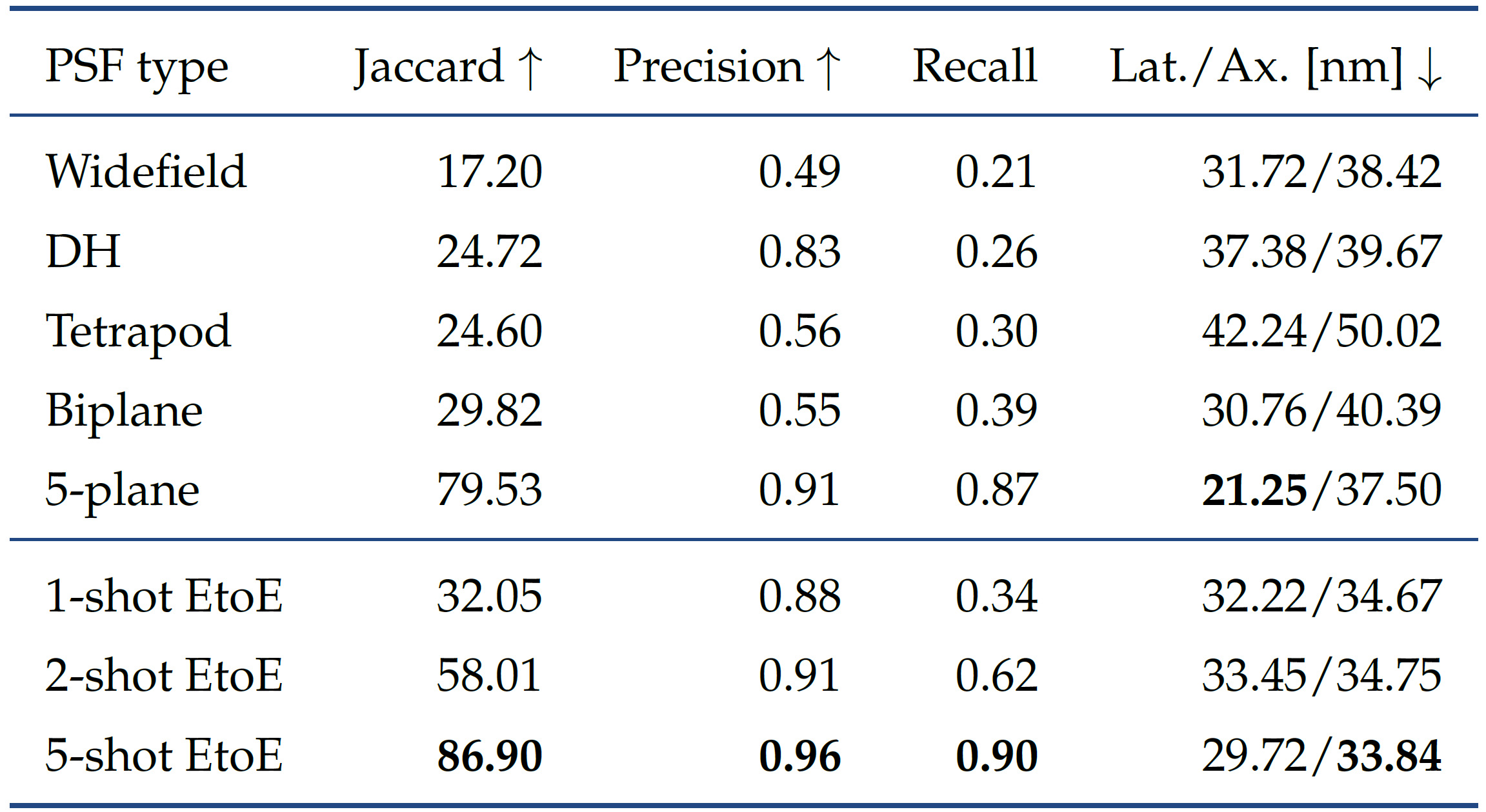
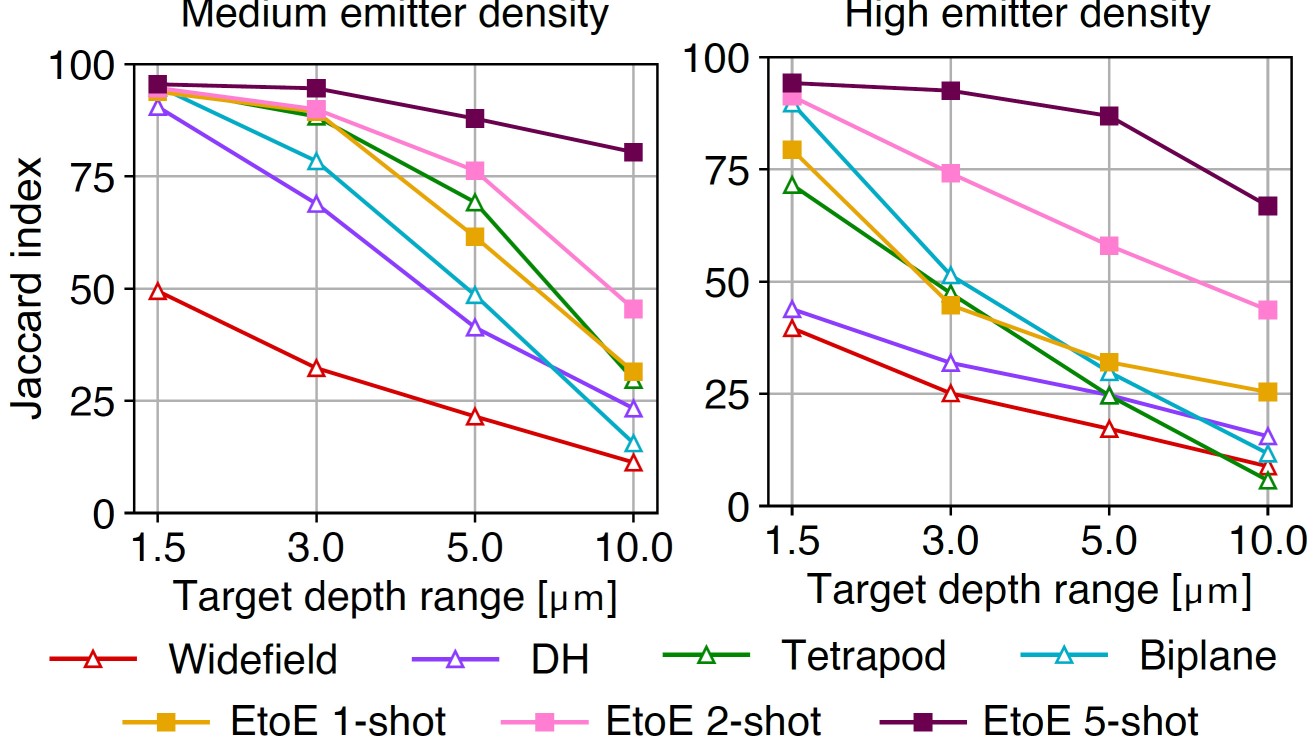
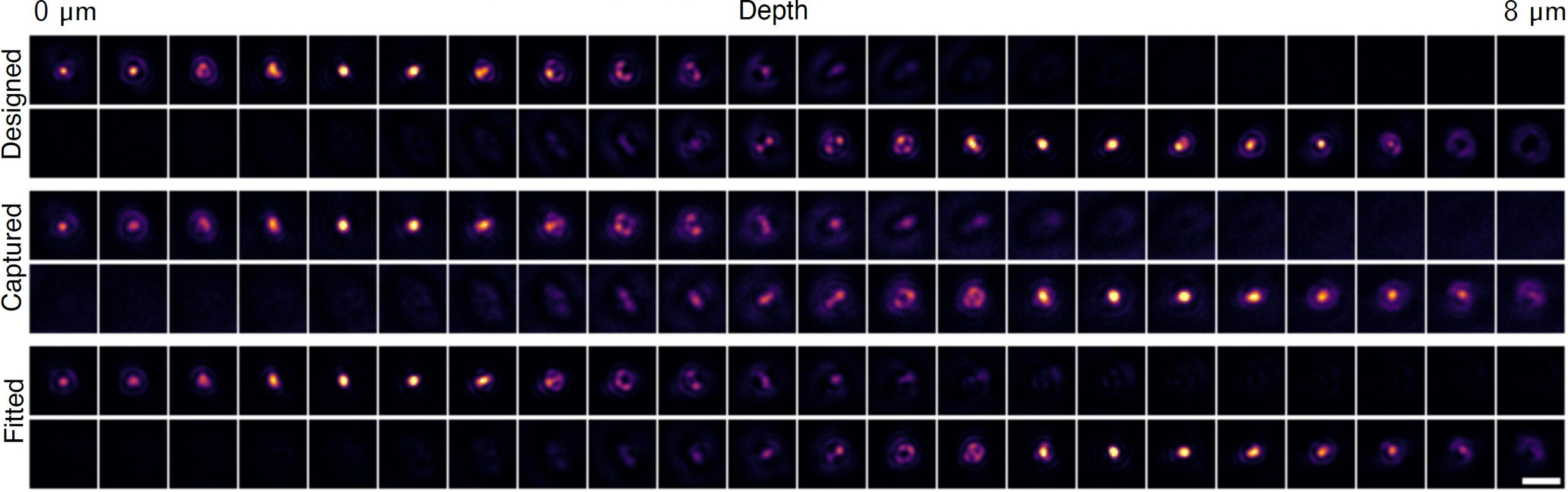
Related Projects
You may also be interested in related projects, where we apply the idea of Deep Optics, i.e. end-to-end optimization of optics and image processing, to other applications
- Ikoma et al. 2021, Depth from Defocus with Learned Optics for Imaging and Occlusion-aware Depth Estimation, ICCP 2021 (link)
- Wetzstein et al. 2020. AI with Optics & Photonics. Nature (review paper, link)
- Martel et al. 2020. Neural Sensors. ICCP & TPAMI 2020 (link)
- Dun et al. 2020. Learned Diffractive Achromat. Optica 2020 (link)
- Metzler et al. 2020. Deep Optics for HDR Imaging. CVPR 2020 (link)
- Chang et al. 2019. Deep Optics for Depth Estimation and Object Detection. ICCV 2019 (link)
- Peng et al. 2019. Large Field-of-view Imaging with Learned DOEs. SIGGRAPH Asia 2019 (link)
- Chang et al. 2018. Hybrid Optical-Electronic Convolutional Neural Networks with Optimized Diffractive Optics for Image Classification. Scientific Reports (link)
- Sitzmann et al. 2018. End-to-end Optimization of Optics and Imaging Processing for Achromatic Extended Depth-of-field and Super-resolution Imaging. ACM SIGGRAPH 2018 (link)
or project that focus on computational fluorescence microscopy
- Kauvar et al. 2020, Cortical Observation by Synchronous Multifocal Optical Sampling Reveals Widespread Population Encoding of Actions, Neuron 2020 (link)
- Ikoma et al. 2018, A convex 3D deconvolution algorithm for low photon count fluorescence imaging, Scientific Reports 2018 (link)
- Prevedel et al. 2014, Simultaneous whole-animal 3D imaging of neuronal activity using light-field microscopy, Nature Methods 2014 (link)
ACKNOWLEDGEMENTS
We thank T. Carver, W.E. Moerner, A. Boettiger, J. Fleischer, M. Gehm, and H. Krovi for fruitful discussions. Part of this work was performed at the Stanford Nano Shared Facilities, supported by the NSF under award ECCS-2026822. This material is based upon work supported by DARPA under Agreement No. HR00112090123. This material is based upon work supported, in part, by the Defense Advanced Research Projects Agency (DARPA) under Agreement No. HR00112090123, a PECASE by the ARO, and Olympus Corporation.